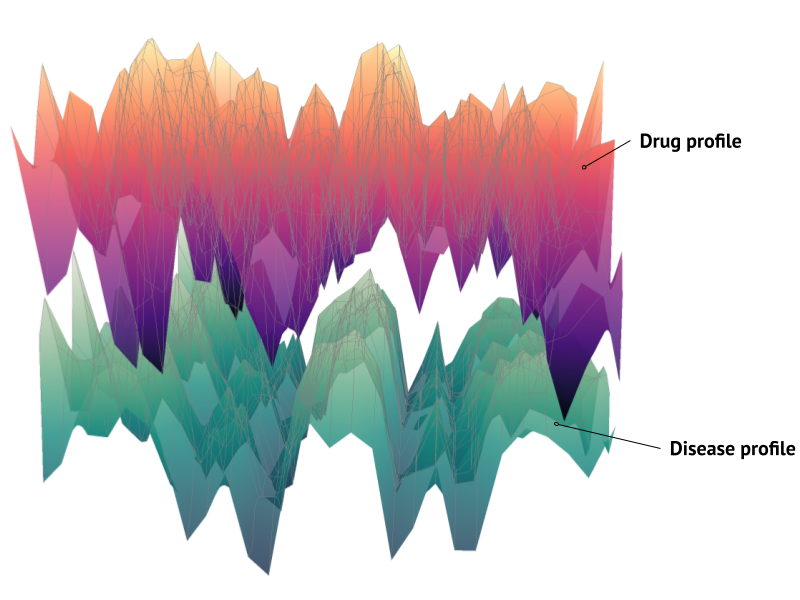Accelerating Drug Discovery with Transcriptomics: A Case Study
Discovering new and viable therapies for genetic diseases is a time-consuming and cost-intensive process, often taking more than a decade and billions of dollars to bring a single new drug to market. For conditions that affect smaller patient populations, the incentives for research and development in the traditional drug discovery lifecycle are often lacking, leaving many patients without hope for a treatment. This reality creates an urgent need for more scalable and efficient solutions. At Transcripta Bio, we’re pioneering a platform that sidesteps the lengthy development of new molecules. By using a high-throughput drug discovery engine, we can uncover how existing, approved drugs impact disease on a cellular level, dramatically accelerating the path to viable treatments.
From a Genetic Anomaly to a Cellular Signature
Our platform’s power is well illustrated by a recent case. We worked with a family whose child was diagnosed with a rare neurodevelopmental disorder, a form of autism spectrum disorder (ASD) linked to chromosomal variants of 19q12. Advanced genetic testing pinpointed the precise cause: a complex genomic rearrangement leading to deficiencies in two critical genes, TSHZ3 and ZNF536, both of which are essential for normal brain development.
To understand the functional impact of this genetic change, we took a small sample of skin cells from the patient and reprogrammed them into neurons in our lab, creating a personalized disease model in a dish. By analyzing the complete set of gene expression patterns, the transcriptome, we gained a comprehensive, 360-degree view of the biological impact of the mutation. This analysis allowed us to identify a clear transcriptomic “disease signature,” a unique fingerprint of cellular dysfunction. In this case, the patient’s neurons showed distinct signs of hyperexcitability, a state where neurons are overly active and fire erratically. This signature became our specific, measurable therapeutic target.
Matching a Disease to a Drug
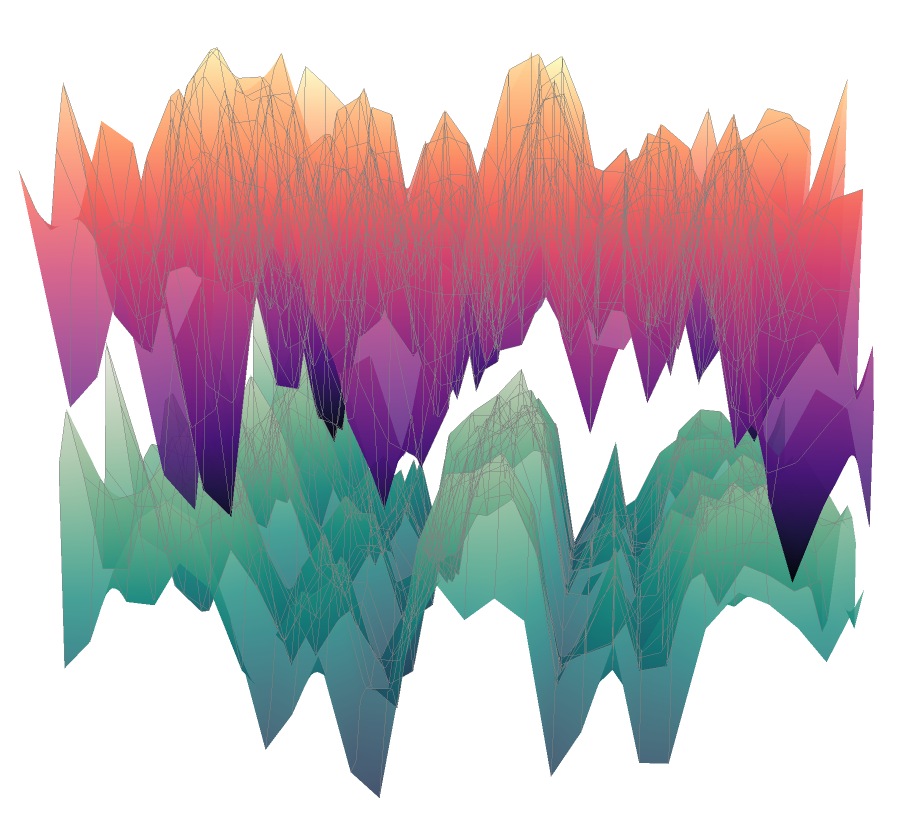
Our core technology is a platform built on two massive, interconnected datasets: a Disease Signature Atlas containing the transcriptomic fingerprints of various diseases, and a Drug-Gene Atlas detailing how thousands of compounds affect gene expression. By cross-referencing these atlases, our goal is to find drugs that can effectively “reverse” or neutralize a specific disease signature.
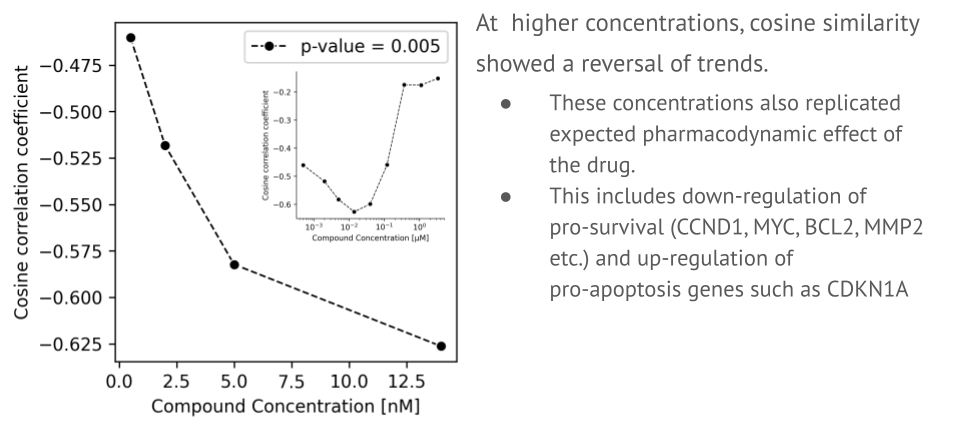
We screened a library of compounds against healthy neurons to map how each one altered gene expression. This computational search allowed us to identify a drug with a transcriptomic profile that was a near-perfect mirror image of the patient’s disease signature. The screen identified a promising candidate: entrectinib, a drug already approved for cancer therapy. This was a critical finding, as repurposing an existing drug can significantly shorten the timeline to clinical use. When we applied entrectinib to our lab-grown patient neurons, it successfully and consistently reversed the hyperexcitability signature
A Personalized Trial and a Promising Outcome
With this compelling preclinical data, and in close partnership with the patient’s physician , an “n-of-1” clinical study was initiated. The patient began treatment with a carefully calibrated low dose of entrectinib under close medical monitoring for safety and efficacy measures.
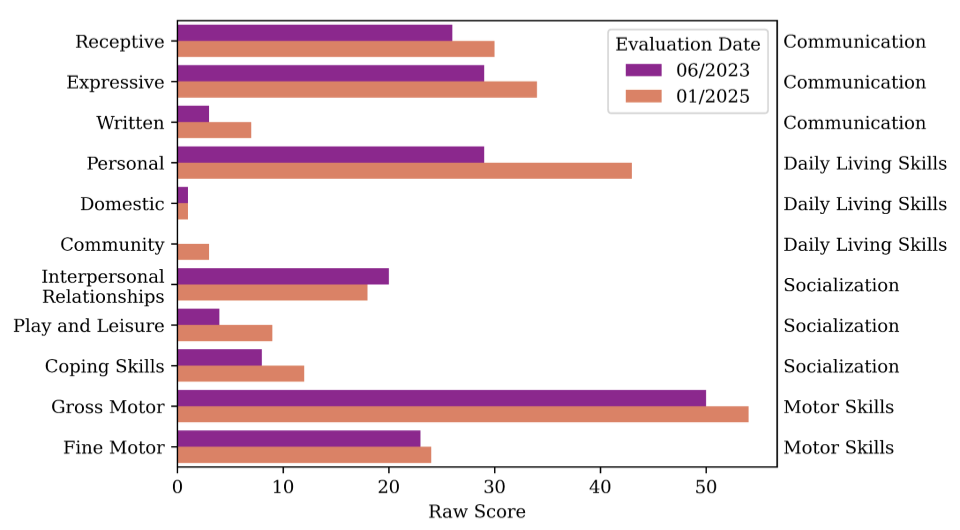
The results have been remarkable. We monitored the patient’s progress by analyzing the transcriptome of whole blood samples collected at regular intervals, which revealed a significant normalization of the disease-related pathways after treatment began. This molecular evidence confirmed the drug was having the intended effect mirroring findings from in vitro neuron treatment. More importantly, these cellular changes translated into real-world benefits, as the patient has shown significant clinical improvements. Standardized developmental assessments, including Vineland scores, have revealed marked improvement in communication and daily living skills, providing a quantitative measure of the treatment’s positive impact.
Looking to the Future
This case is more than just a single success story; it’s a blueprint for a new way of discovering therapies for genetically-defined diseases. We’ve demonstrated that by deeply understanding the molecular basis of a disease, we can use transcriptomics to rapidly match patients with potentially life-changing treatments. This platform-based approach is adaptable and scalable.
We are already extending this discovery pipeline to other complex disease areas and exploring the underlying mechanisms of how these therapies work. Our work with entrectinib suggests that it helps rebalance synaptic activity, offering potential avenues for treating other neurological disorders characterized by hyperexcitability.
We are immensely grateful to the family, their physicians, and the philanthropic partners who made this research possible. Their collaboration was essential to this breakthrough. Together, we are paving the way for a future where every patient with a genetically-defined disease has a clear and rapid path to a personalized therapy.